New Vaccine Candidate Holds Promise to Conquer Malaria
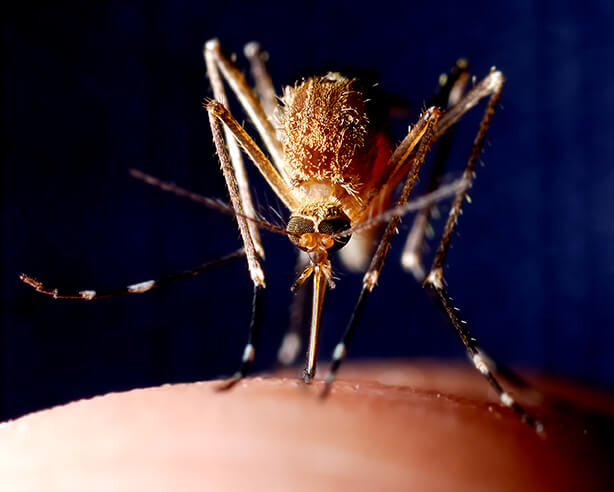 March 28, 2024 – Researchers in Seattle Children’s Research Institute’s Center for Global Infectious Disease Research (CGIDR), in collaboration with the biotechnology company Sanaria, used genetic engineering to develop a novel malaria vaccine called PfSPZ-LARC2 that has the potential to confer high levels of protection against malaria infection and as such, could prevent the death of hundreds of thousands of children and help eliminate malaria. The findings were recently published in the journal EMBO Molecular Medicine. The journal concurrently published a News & Views article about the paper.
March 28, 2024 – Researchers in Seattle Children’s Research Institute’s Center for Global Infectious Disease Research (CGIDR), in collaboration with the biotechnology company Sanaria, used genetic engineering to develop a novel malaria vaccine called PfSPZ-LARC2 that has the potential to confer high levels of protection against malaria infection and as such, could prevent the death of hundreds of thousands of children and help eliminate malaria. The findings were recently published in the journal EMBO Molecular Medicine. The journal concurrently published a News & Views article about the paper.
The deadliest form of malaria is caused by Plasmodium falciparum parasites transmitted by mosquitoes. The parasite grows in mosquitoes, colonizes in the salivary glands, and then is transmitted by bite to humans, where it asymptomatically infects the liver, replicates, merges into the bloodstream and infects red blood cells, causing disease and sometimes death.
The World Health Organization estimates there were 249 million malaria cases and 608,000 malaria deaths in 85 countries in 2022. In Africa, which experiences a disproportionately high share of the global malaria burden, children under 5 accounted for about 80% of all malaria deaths.
“Through dual gene deletion, we have created the PfSPZ-LARC2 parasite vaccine that can multiply in the liver without causing any malaria symptoms. This stimulates a very strong immune response against the parasite, protecting against future malaria infections,” said Dr. Stefan Kappe, the CGIDR principal investigator who led the study. “But then the LARC2 vaccine disintegrates in the liver, which prevents new parasites from getting into the bloodstream.”
The study team demonstrated the human vaccine’s safety by testing LARC2 — which was engineered to lack two parasite genes, Mei2 and LINUP — in a humanized animal model with human liver cells and human red blood cells. In a conventional animal model, the vaccine gave 100% protection against infection.
A Second-Generation Vaccine
Presently, there are two approaches to malaria vaccine development. One is a subunit approach, where individual proteins (the parasite has about 5,500) are expressed in a recombinant system. RTS,S/AS01 and R21/Matrix-M subunit vaccines — currently the only approved malaria vaccines — target a single parasite species and a single parasitic antigen, which may result in the emergence of parasites that evade vaccine-induced immunity. They offer moderate protection against the disease but protection against infection remains unknown. These vaccines do not prevent further transmission of the parasite to the mosquito vector and thereby, do not halt the spread of the malaria parasite.
Kappe and colleagues consider the subunit method in its current form of limited use for malaria elimination. They have focused on developing a second-generation vaccine with improved characteristics to have greater impact, capable of blocking P. falciparum infection, reducing transmission and potentially protecting against multiple parasite species.
By using a live attenuated (weakened) approach, which employs the whole organism as a vaccine, the researchers target a broader range of antigens than subunit vaccines. This has consistently been shown in animal studies to not only prevent the clinical symptoms of malaria but also to block infection and, thus, cease further transmission.
Upcoming Clinical Trials
Kappe said if all goes well with Food and Drug Administration’s Investigational New Drug approval, the PfSPZ-LARC2 vaccine will be assessed in clinical safety and efficacy trials beginning this summer and running through 2025 in the U.S., Germany and Burkina Faso.
“It took us nearly 20 years to get to the PfSPZ-LARC2 vaccine,” said Kappe, who is also a professor of pediatrics at the University of Washington School of Medicine. “Finding the right genes — that when deleted from the parasite genome, stop the parasite at the point when it wants to get out of the liver — was an enormous task. In a nutshell, LARC2 can check into the liver but it cannot check out. Our results provide a clear indication that safe, complete developmental attenuation of malaria parasites is possible in the liver, and we are excited to see the outcome of the first clinical trials with this vaccine.”
“This vaccine has the potential to save millions of lives and eliminate malaria from defined geographic regions when administered in mass vaccination programs,” said Dr. Stephen Hoffman, Sanaria founder and CEO. “We are very enthusiastic about this novel, genetically engineered vaccine strain. Sanaria has already manufactured the PfSPZ-LARC2 vaccine, and we look forward to the results from clinical trials.”
Vaccine Award Finalist
The efforts of the Kappe-Sanaria partnership have not gone unnoticed by the global vaccine community. Their vaccine is one of six finalists for best prophylactic vaccine at the annual Vaccine Industry Excellence (ViE) Awards, taking place on April 2 in Washington, D.C., as part of the World Vaccine Congress meeting. These awards have been created to honor and generate recognition of the efforts, accomplishments and positive contributions of companies and individuals in the vaccine industry. “It’s like an Oscar nomination for vaccines,” Kappe said.
The Kappe Lab’s Debashree Goswami and Hardik Patel were co-first authors of the paper, and the lab’s Will Betz, Janna Armstrong and Nelly Camargo also contributed. Additional authors include Dr. Ashley Vaughan, CGIDR principal investigator and assistant professor of pediatrics at the University of Washington School of Medicine, who is a critical collaborator on this vaccine approach, and Dr. Sean Murphy, associate professor of laboratory medicine and pathology at the University of Washington School of Medicine.
The research was funded by NIH’s National Institute of Allergy and Infectious Diseases and by the Small Business Innovation Research program.
— Colleen Steelquist
