Fishing for Answers in Drug-Induced Hearing Injury
 March 21, 2024 – Researchers in Seattle Children’s Research Institute’s Center for Global Infectious Disease Research (CGIDR) and colleagues have developed a new platform to screen for adverse drug interactions that aggravate or protect against drug toxicity. The platform, which uses zebrafish as a model organism, revealed a drug interaction between two common types of antibiotics that protected against medication-induced hearing injury. Their findings were published in the journal Frontiers in Pharmacology.
March 21, 2024 – Researchers in Seattle Children’s Research Institute’s Center for Global Infectious Disease Research (CGIDR) and colleagues have developed a new platform to screen for adverse drug interactions that aggravate or protect against drug toxicity. The platform, which uses zebrafish as a model organism, revealed a drug interaction between two common types of antibiotics that protected against medication-induced hearing injury. Their findings were published in the journal Frontiers in Pharmacology.
“This research tells us that toxicity protection does not have to undermine the antibiotics’ ability to effectively target bacteria, which means we should be able to develop strategies that can simultaneously optimize protection against toxicity and improve efficacy,” said Dr. Shuyi Ma, a CGIDR principal investigator who primarily studies tuberculosis (TB).
Ototoxicity as a Side Effect
Over 150 medications, including aspirin and ibuprofen, can potentially damage hearing as a side effect — known as ototoxicity — when used singly or in combination with other medicines, but it’s more common with certain antibiotics and with cancer drugs like cisplatin. Currently, many of these drug interactions are discovered only after the medicines are in use, as drug effects on the ear are not routinely tested in preclinical and clinical trials.
For TB patients with hard-to-treat infections who are treated with aminoglycoside antibiotics like streptomycin or gentamicin, permanent drug-induced hearing loss occurs in up to one-third of patients. Researchers have found people who are exposed to loud noise for extended periods of time are especially sensitive to this kind of injury.
“Current use of aminoglycoside antibiotics is really limited because of the side effects,” Ma said. “That’s true not just for TB, but for other hard-to-treat infections, like in patients with cystic fibrosis (CF). There is a need to make our arsenal of drugs more tolerable so they’re safe for patients.
“With TB, permanent drug-induced hearing injury occurs in part because of how long you need to take those drugs to treat those infections. The severity and permanence of the side effect is proportional to how long and how much you take, so when patients have to take the drugs for months at a time in very hard-to-treat infections, it becomes a really big problem,” she said.
Ma, who is also an assistant professor in the departments of Pediatrics, Chemical Engineering, and Global Health at the University of Washington, said addressing toxicity and alleviating side effects will help researchers and providers figure out how to better use available antibiotics.
Team Science
For this research study — the first led by Ma — she and her team collaborated with the Center for Clinical and Translational Research’s Dr. Henry Ou, an otolaryngologist and director of Seattle Children’s Hearing Loss Clinic, and Dr. Rafael Hernandez, a CGIDR principal investigator.
Zebrafish have been used historically to model toxicities in many different organs because they're both genetically tractable and easy to image. Ma credits the Hernandez Lab with introducing her to the world of zebrafish toxicology. Ou and University of Washington’s Dr. David Raible are pioneers of using zebrafish as an animal model for drug-induced hearing injury. Both offered guidance to the Ma Lab in setting up the model, and the Raible Lab contributed one of the transgenic fish lines used in the study. “Collaborative science is the best kind,” Ma said.
Toxicity is a complex phenomenon, and sometimes the effects are not the product of just damage to an individual cell type, noted Ma. “It's the collective activity of multiple cells, tissues or organs working together, so having a model that would enable us to look more holistically was useful. And with a single pair of adult fish, you can generate hundreds of larvae per week,” she said. “We imagine developing a customized fish that would allow us to look at multiple organ toxicities to help guide drug development in the future.”
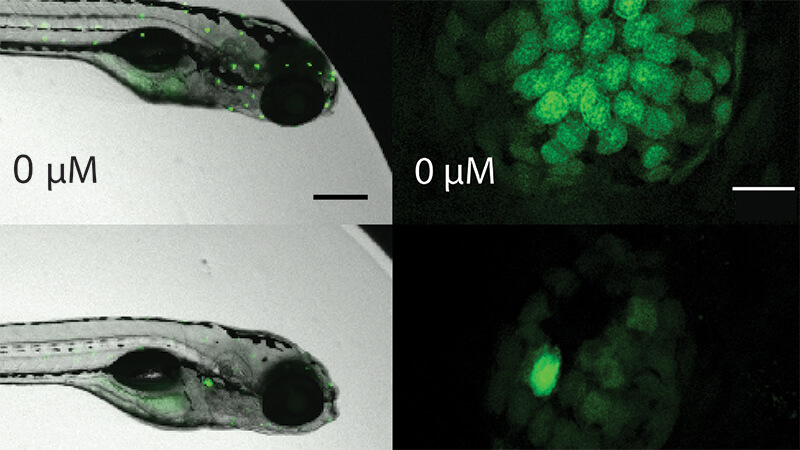
For the study, Ma and her lab colleagues developed a novel workflow, Parallelized Evaluation of Protection and Injury for Toxicity Assessment (PEPITA), which enabled them to measure ototoxicity and protection against ototoxicity in hundreds of zebrafish larvae at a time via robotics-assisted microscopy. They were able to characterize multiple drug interactions by observing cellular damage to the zebrafish lateral line hair cells, which are structurally and functionally like the human inner ear.
They found a combination of macrolide (like azithromycin) and aminoglycoside antibiotics (like gentamicin) protected against aminoglycoside-induced damage to lateral line hair cells in the zebrafish larvae. This toxicity protection did not impair the antibiotics’ ability to kill disease-causing bacteria. The mechanism of toxicity protection appears to be different from other treatments that protect against hearing injury-associated damage.
Ma said the platform her team developed is amenable to screening any kind of drug that could injure hearing, so they are interested in looking at other categories of medications.
“We want to find out the extent to which the protection that we observe in zebrafish translates to toxicity protection in mammals and humans,” Ma said.
Future Studies
To that end, Ma is planning to collaborate with the Center for Respiratory Biology and Therapeutics’ Dr. John Cogen, a clinician-researcher who specializes in patient health record studies.
“We plan to see if existing patient health records can give us some indication of whether treating with this drug combination is protective because both of these drug classes are already approved to treat really serious infections in CF patients,” Ma said. “Potentially, they might already be helping those patients.”
There is a strong molecular connection between drug-induced hearing injury and drug-induced kidney damage. Ma also wants to learn if the protection they observed could also protect against drug-induced kidney damage.
Most importantly, Ma and her team want to know how the protective drug interaction they discovered works to protect against drug-induced hearing injury. “Specifically, we want to find out what these cellular drug targets are that confer the protection, how they act with the drug combination to protect whilst retaining antibacterial efficacy, so that we can figure out how we might design more potent interventions to improve on level of toxicity protection conferred,” she said.
Additional contributing authors from Seattle Children’s include the Ma Lab’s Ethan Bustad, Emma Mudrock (now in IACUC/IBC) and Elizabeth Nilles. Former staff members who contributed to the research include Monica Bergado, Alden Gu, Louie Galitan and Natalie Gleason.
The research was funded by the National Institutes of Health and the Lura Cook Hull Trust.
— Colleen Steelquist
