Discovering Patterns in the Neural Activity Powering Breathing
 Jan. 19, 2024 – Researchers in Seattle Children’s Research Institute’s Norcliffe Foundation Center for Integrative Brain Research (NFCIBR) used novel neural recording technology to observe thousands of brainstem neurons, allowing identification of the network activity patterns underlying breathing. Their findings mark a first-ever population-level analysis of large recordings of brainstem neurons in breathing centers.
Jan. 19, 2024 – Researchers in Seattle Children’s Research Institute’s Norcliffe Foundation Center for Integrative Brain Research (NFCIBR) used novel neural recording technology to observe thousands of brainstem neurons, allowing identification of the network activity patterns underlying breathing. Their findings mark a first-ever population-level analysis of large recordings of brainstem neurons in breathing centers.
The study, published in the journal Nature Neuroscience, offers new behavioral insights because it studies how individual, identified breathing neurons work together as a population to generate breathing in the brainstem. Looking at the dynamic properties of these large populations of neurons provided new insights never before obtained by looking at the interactions of small groups of individual neurons.
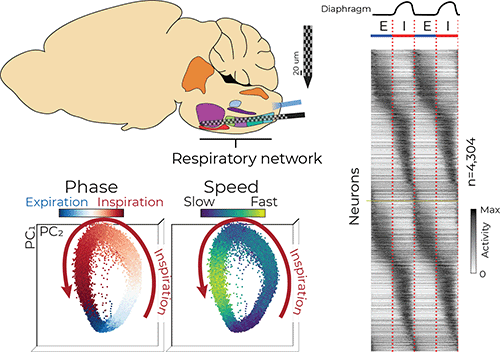
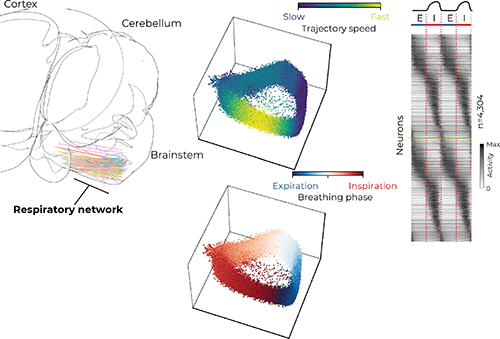
Several regions in the brainstem, collectively called the ventral respiratory column (VRC), are critical for generating and maintaining breathing. Dr. Jan-Marino “Nino” Ramirez and Dr. Nicholas Bush, a postdoctoral fellow in the Ramirez Lab, surveyed neural activity in the VRC using cutting-edge Neuropixels probes, which are smaller than a human hair and have approximately 1,000 electrode locations that can record the electrical activity of more than 100 neurons simultaneously with sub-millisecond precision.
 At the same time, the researchers used a technique called “opto-tagging” to determine whether the neurons they recorded were inhibitory (silencing) or excitatory (stimulating) with the neurons to which they were communicating. Using a genetically engineered small-animal model with only one type of neuron that can be activated by blue light, the neurons were identified as inhibitory or excitatory while the neural activity was recorded.
At the same time, the researchers used a technique called “opto-tagging” to determine whether the neurons they recorded were inhibitory (silencing) or excitatory (stimulating) with the neurons to which they were communicating. Using a genetically engineered small-animal model with only one type of neuron that can be activated by blue light, the neurons were identified as inhibitory or excitatory while the neural activity was recorded.
“It is well known that individual neurons are active at specific parts of the breathing cycle, such as during inspiration [inhalation],” Bush said. “It has largely been thought that these neurons form groups which have similar activity patterns, but the groups are distinct from each other. Our large dataset revealed these groups actually smear into each other to create a continuum of respiratory neuron types.”
The researchers used computational tools to investigate the coordinated activity of the simultaneously recorded neurons. Similar to birds in flight — the flock moves as one but each bird is flying independently — the researchers analyzed the activity of hundreds of neurons and described their movement as a population.
“We saw the population as a whole rotates around in a circle like a racecar on a track,” Bush said. “Interestingly, these populations tend to move fastest during inspiration, and shoot toward a ‘finish line’ at the end of inspiration before starting another lap.” He said this observation highlights the transition between inhalation and exhalation as a reorganizing feature that may be important for other behaviors that rely on breathing, like swallowing and vocalizing.
In addition to looking at how neural populations behave during normal breathing, the research team sought to understand what happens when breathing is disrupted.
One form of disrupted breathing and a leading cause of death in the U.S. is opioid induced respiratory depression (OIRD), the slow and shallow breathing caused by opioid overdose. There is still limited understanding of the molecular, cellular and circuit mechanisms of OIRD. The researchers used opioids in the small-animal model and observed the respiratory network activity. They found the “racetrack” rotation did not go away, but slowed down as if there was a new, lower speed limit. This suggests the neural network has redundancies to compensate when breathing is disrupted to preserve breathing function.
Lowering the oxygen levels given to the small-animal model — similar to the conditions believed to cause SIDS — altered the normal breathing pattern and caused gasping.
“We found the racetrack rotations eventually gave way to something more like a drag strip. The populations of neurons would sit in one spot between breaths, then shoot out and back during large gasps,” Bush said. “This drastic change in the behavior of the population highlights the reorganization of the brainstem activity that happens during gasping.”
This basic science work furthers understanding of the brain circuitry that underlies breathing and may guide future research into how that circuitry goes awry in conditions like epilepsy and SIDS.
The work was supported by grants from the National Institutes of Health and by the research institute’s Research Scientific Computing.
— Colleen Steelquist
