Miao Lab
Developing innovative hemophilia treatments
Dr. Carol Miao is pursuing safer, more effective treatments for hemophilia. Her lab focuses on developing innovative gene therapy and immunotherapy approaches. Several approaches that could potentially be translated into real-world treatments for children and adults with hemophilia are currently under investigation. The projects include:
Nonviral gene delivery for hemophilia
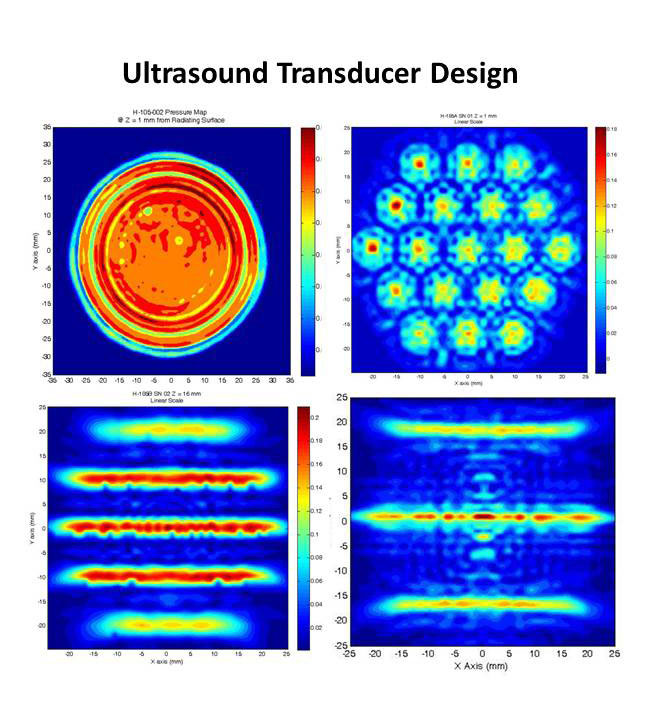 Although viral gene therapy had success in animal models and recent clinical trials, many obstacles remain. Our lab previously demonstrated that ultrasound (US) mediated gene therapy method can significantly enhance reporter gene transfer into mouse and rat livers. This nonviral gene transfer strategy can bypass many obstacles encountered by viral gene therapy.
Although viral gene therapy had success in animal models and recent clinical trials, many obstacles remain. Our lab previously demonstrated that ultrasound (US) mediated gene therapy method can significantly enhance reporter gene transfer into mouse and rat livers. This nonviral gene transfer strategy can bypass many obstacles encountered by viral gene therapy.
Most significantly, we have recently achieved therapeutic levels of factor VIII (FVIII) following US-mediated gene delivery into hemophilia A mice. In order to facilitate the eventual translation of these technologies into human application, we have developed ultrasound systems and new generations of transducers to successfully apply ultrasound technology for treating large tissue volumes in large animal models including dog and pigs.
Recently, we have also synthesized and screened for the best custom-made, targeted microbubbles to further improve gene transfer efficiency. These projects will facilitate the translation of this novel technology into human application, and could change fundamentally the way hemophilia patients are treated, with better patient outcomes. We hope to explore the possibility of clinical trials using this new technology.
Drug and cell immunotherapy for modulating immune responses following gene and protein replacement therapy
Fully 30% of hemophilia A patients develop inhibitor antibodies against factor VIII following protein replacement therapy. Current tolerance induction protocol involves long-time administration of high doses of factor VIII, which is both inconvenient and very costly. What’s more, one third of the patients fail to induce tolerance using this protocol. Prophylactic tolerance induction protocols involving a short immunosuppressive regimen with minimum side effects and toxicity are highly promising, alternative strategies for patients at high risk.
Our lab has developed several potent immunotherapies to prevent inhibitory antibody production in hemophilia A mice, including agents to block costimulatory pathways such as CTLA4-Ig combined with anti-CD40L, and anti-ICOS, agents to deplete T cells such as anti-CD3, or agents to induce Treg cell expansion such as IL2-IL2mAb complexes, which can be administered in combination with repeated injections of low doses of FVIII to induce long-term tolerance of FVIII. These new transient immunosuppression strategies for tolerance induction can not only reduce the costs, but also shorten the treatment time and increase the success rate. Clinical testing of some of these regimens is highly anticipated.
In addition, it has recently been demonstrated that CD4+CD25+Foxp3+ regulatory T cells (Treg) play a critical role in the regulation and suppression of autoimmune and alloimmune responses. Our preliminary data showed that an adoptive transfer of transgenic CD4+Foxp3+ Treg cells significantly reduced anti-FVIII antibody titers in FVIII plasmid-treated HemA mice. Thus, we have embarked on regulatory T cell therapy projects, including in vitro expansion of antigen-specific Treg cells, gene editing technology in collaboration with other investigators in the Center for Immunity and Immunotherapies, as well as chimeric antigen receptor (CAR)-Treg cell therapy for antigen-specific regulation of FVIII-specific immune responses.
Lentiviral gene therapy for hemophilia
Intraosseous (IO) infusion of lentiviral vectors (LVs) for in situ gene transfer into bone marrow may avoid specific challenges posed by ex vivo gene delivery, including, in particular, the requirement of pre-conditioning. We have successfully transduced a high percentage of primitive hematopoietic stem cells (HSCs) in bone marrow following IO delivery of high-titer LVs. Furthermore, by IO delivery of G-F8-LV encoding a factor VIII (FVIII) transgene directed by a platelet-specific, glycoprotein-1bα promoter, we achieved long-term platelet-specific expression of FVIII, resulting in partial correction of hemophilia A. Similar clinical benefit with G-F8-LV was achieved in animals with pre-existing anti-FVIII inhibitors. These findings further support platelets as an ideal FVIII delivery vehicle, as FVIII, stored in α-granules, is protected from neutralizing antibodies and, during bleeding, activated platelets locally excrete FVIII to promote clot formation. Overall, a single IO infusion of G-F8-LV was sufficient to correct hemophilia phenotype for long term. We are currently improving further the constructs and delivery protocols. We would like to push this new methodology for clinical trials in the near future.
Latest News
April 29, 2024
Seattle Children’s Researchers Highlight Achievements in Novel Therapies at Gene and Cell Therapy Meeting (Seattlechildrens.org)
Key findings from the Center for Immunity and Immunotherapies will be shared at the American Society of Gene and Cell Therapy’s Annual Meeting from May 7 to 11 in Baltimore, Maryland.
May 28, 2023
New Gene Therapy Delivery Methods (CGTlive.com)
Gene therapy researcher Dr. Carol Miao and team evaluate nonviral genomic medicines.
May 26, 2023
Gene Delivery and Editing to Treat Hemophilia (CGTlive.com)
Dr. Carol Miao shares how lipid nanoparticles carrying gene-editing tools can treat bleeding disorders.
Selected Publications
- Miao CH, Ye P, Thompson AR, Rawlings DJ, Ochs HD. (2006) (Plenary paper) Immunomodulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice, Blood, 108, 19-27. PMCID: PMC1895820
- Miao CH, Harmeling BR, Ziegler SF, Yen BC, Torgerson T, Chen L, Yau RJ, Peng B, Thompson AR, Ochs HD, Rawlings DJ. (2009) CD4+FOXP3+ regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice, Blood, 114(19):4034-44. PMCID: PMC2774545
- Peng B, Ye P, Rawlings DJ, Ochs HD, Miao CH. (2009) Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy, Blood, 114(20):4373-82. PMCID: PMC2777123
- Song S, Noble M, Sun S, Chen L, Brayman AA, Miao CH (2012) Efficient microbubble- and ultrasound-mediated plasmid DNA delivery into a specific rat liver lobe via a targeted injection and acoustic exposure using a novel ultrasound system, Mol Pharm. 9(8), 2187-2196.
- Noble ML, Kuhr CS, Graves SS, Loeb KR, Sun SS, Keilman GW, Morrison KP, Paun M, Storb RF, Miao CH. (2013) Ultrasound-targeted microbubble destruction-mediated gene delivery into canine livers, Mol Ther. 21(9):1687-94. PMCID:PMC3776626
- Sun RR, Noble ML, Song S, Sun SS, Miao CH. (2014) Development of therapeutic microbubbles for enhancing ultrasound-mediated gene delivery J Control Release. 182:111-20.
- Liu C-L, Ye P, Lin J, Djukovic D, Miao CH. (2014) Long-term tolerance to factor VIII is achieved by administration of IL-2/IL-2mAb complexes and low dosages of factor VIII, J Thromb Haemost. 12(6):921-31. PMCID:PMC4055525
- Wang X, Shin SC, Chiang AF, Khan I, Pan D, Rawlings DJ, Miao CH. (2015) (Featured Article on Mol. Ther. Website) Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A, Mol Ther. 23(4):617-26. PMCID:PMC4395783
- Liu CL, Lyle MJ, Shin SC, Miao CH.(2016) “Strategies to target long-lived plasma cells for treating hemophilia A inhibitors, Cell Immunol. 301:65-73. PMCID:PMC4844017
See a complete list of Miao’s publications on PubMed.
Investigator Biography

Carol H Miao, PhD
Carol Miao, PhD, is a principal investigator in the Center for Immunity and Immunotherapies at Seattle Children’s Research Institute, and a professor in the Department of Pediatrics at the University of Washington.
